Posted on Aug 5, 2012 in News |
The effects of suboptimal maternal haemoglobin levels on perinatal outcome have been well studied in numerous cohorts around the world. Child-bearing women who are anaemic (defined as a haemoglobin level of less than 11 grams per decilitre) are at risk of preterm delivery, having babies of low birth weight, neonatal APGAR scores of less than 5 at 1 minute and most significantly are at increased risk of fetal death in utero.[1] The prevalence of anemia (most commonly iron deficiency anemia) is highest among pregnant women due to the increasing demands of the growing fetus. It has been estimated that 49% of pregnant women and 45% of children under five are affected by iron-deficiency anemia, which equates to approximately 150,000 maternal and 700,000 newborn deaths annually.[2]
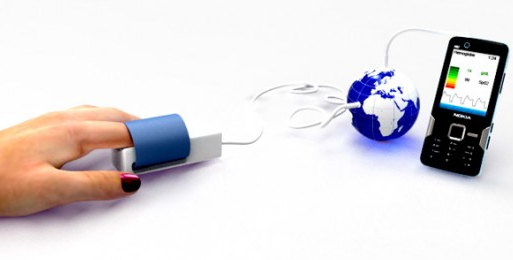
A group of biomedical engineering undergraduate students at Johns Hopkins University in Baltimore, America have possibly contrived the solution for global maternal anemia: Hemoglobe, which is a non-invasive device connected to a mobile phone to estimate the haemoglobin level of the user. With the burgeoning use of mobile phone devices amongst health care workers in remote locations around the globe[3], the detection of pregnant mothers with anaemia is a close reality with field testing of this new device planned for communities in Kenya early next year. Hemoglobe functions by the principle of absorption spectrophotometry: the sensor is placed over the patient’s fingertip and different wavelengths of light are emitted, which are then absorbed by the red blood cells in the capillaries. The device then measures the degree of light absorption and this transmits to a program on the mobile device to calculate the haemoglobin level.
Estimated costs to produce the device are around the vicinity of US 10 to 20 dollars. The technology is not new as other medical device companies such as Masimo have developed similar devices, however the software connected to the HemoGlobe will also send an automated message to a clinical centre. Thus health workers are able to determine which areas have the highest prevalence and direct resources accordingly from something as simple as iron supplementation to expediting a review at the nearest clinic. As one of the developers Greenbaum states, the technology is now functional, but: “now, we have a greater challenge: to prove that it can have a real impact by detecting anemia and making sure the mothers get the care they need.”
[2] Mason, Rivers and Helwig. “Recent trends in malnutrition in developing regions: Vitamin A deficiencies, anemia, iodine deficiency, and child underweight,” Food and Nutrition Bulletin 26: 57-162, 2005.
[3] Tamrat T. ‘Special delivery: an analysis of mHealth in maternal and newborn health programs and their outcomes around the world.’ Maternal Child Health Journal. 2011 Jun. (online)
Posted on Jul 11, 2012 in News |
A low-cost digital stethoscope that can attach to a smartphone may represent the future of saving millions of children in Third world countries. StethoCloud is is designed to listen and digitalise a patient’s breathing sounds and patterns. Those patterns are then compared against a medical database via cloud infrastructure. Then an automated report is generated though Algorithmic Artificial Intelligence Decision Support. Such software could potentially allow an earlier diagnosis of pneumonia and reduce the mortality of children in developing countries.
StethoCloud is the Australian entry in the international Imagine Cup 2012. The Australian software design team led by Hon Weng Cheng, a third year medical student at the University of Melbourne, has advanced to the second round and is in the World wide finals. Go Aussies! Oi-oi-oi!!
SteathoCloud at the Imaginecup
Posted on Jul 8, 2012 in News |
Medical devices that combine consumer electronics and apps are revolutionizing chronic disease management. These new breeds of medical devices interface with smartphones to allow patients with chronic diseases, such as diabetes, to monitor their health and share the information with their treating physicians. Mobile disease management has the potential to collect, organize and analyze patient data through evidence-based clinical guidelines, and then give patients feedback when they need it. Furthermore it can connect patients to their health care providers, educators and other people who have the same disease.
Diabetes affects 171 million people worldwide and it is estimated by the year 2030, 366 million people worldwide will have diabetes[1]. There is an increasing burden on primary care physicians and endocrinologists to manage patients with diabetes, and consequently patient care will be compromised. On average a typical patient with diabetes will spend 6 hours a year with their primary care provider. Then they are left to manage a complex and challenging disease on their own. Mobile disease management can fill in this “in-between” time.
One such device is iBGStar, which was developed by Sanofi and AgaMatrix. iBGStar is a glucose meter that can be used on its own, or connected directly to an iPhone or iPod touch. The information stored on the app can be shared with the patient’s healthcare team. When the patient goes to the doctor, she can see pattern of the blood glucose readings and adjust medication or advise behavior changes accordingly.
WellDoc, a Baltimore-based behavioral science and technology company, developed a super-app called DiabetesManager. DiabetesManager collects data on the patient’s blood glucose levels, diet, exercise and medication regime, then uploads information to the cloud-based analytic system. The system provides automated feedback to the patient based on that data in the form of alerts, prompts and positive reinforcement. They recently published their results and showed that there was a statistically significant 1.9% mean decrease in A1c levels for the intervention group who used the DiabetesManager compared to a 0.7% mean decrease for the control group over a 12 month period[2].
Mobile disease management will provide a more diversified platform that will enable constant connectivity between patients and their doctors. It will not be long before this field expands to multiple therapeutic areas and a number of other chronic diseases such as COPD, heart disease and pain management.
[1] World Health Organization, “Facts and figures about diabetes,” http://www.who.int/diabetes/facts/world_figures/en/
[2] Quinn CC, Shardell MD, Terrin ML et al., Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control, Diabetes Care, 2011; 34(9):1934-42.
Posted on Jul 1, 2012 in News |
Human communication contains much information that is not verbalized. Facial expressions, facial micro-expressions, hand gestures and speech patterns are all signals of who we are. There is a new wave of startups and entrepreneurs looking to make an impact in healthcare. Companies such as Cognito and Affectiva are developing innovative mobile technology designed to analyze emotions, studying vocal and visual clues as well as physiological factors. The premise of this new technology measuring human emotion is to focus on how people speak and interact, not what they are saying.
New technology developed by Affectiva began as collaborative research effort at the MIT Media Lab to help people on the autism spectrum who have difficulty reading emotion. Now the Affdex emotion measurement technology, which reads emotional states such as smiling, confusion, dislike, has been commercialized to help businesses understand their customers by quantifying the emotional connection consumers have to brands. Cognito has been developing “Honest Signals” technology that measures patient engagement through vocal clues, either through phone conversations or face-to-face meetings.
Emotional measurement can be beneficial in many areas such as marketing research and clinical research. However, an area that this new technology may have a significant impact is mental health. Mental health disorders affect millions of people worldwide. According to the World Health Organisation1, mental health disorders account for 13% of the global burden of disease, and is poorly recognized. The treatment gap for mental disorders is large all over the world. In low- to middle-income countries, between 76% and 85% of people with severe mental disorders receive no treatment for their mental health conditions. The corresponding figures for high-income countries are also high – between 35% and 50%.
Measuring patients’ emotions allows healthcare professionals to pick up signs that a patient might not recognize or be willing to express. The application of such technology may be useful to diagnose mental health disorders and post-traumatic stress disorders, and even helping occupational healthcare providers to spot stress in employees and preventing burnout.
Emotional measurement technology is still very experimental and much validation work needs to be done before it can be applied to healthcare. There may come the day when a patient’s mood is measured with a psychological sensor that is as reliable as a blood pressure cuff!
1. World Health Organization, Sixty-fifth World Health Assembly, May 12, 2012. http://www.who.int/mental_health/WHA65.4_resolution.pdf
Posted on Jun 7, 2012 in News |
The Editorial Board at the Journal of Mobile Technology in Medicine is proud to present Volume 1, Issue 2, published on June 1st 2012. After our inaugural publication in March 2012, we have had considerable interest from researchers in the field, and received a number of submissions. Mobile technology in Medicine is a rapidly developing area, and we hope to continue accelerating research in the field. We look forward to your submissions for Issue 3
Volume 1, Issue 2 Contents
Editorials
001 Validate an App: How to Design Your Study and Get Published
O. Franko
005 Principles of Security for the use of Mobile Technology in Medicine
C. Perera
Letters
008 An experience of the virtual desktop: a surgical perspective
P Boekel
Original Articles
011 Mobile Technology Usage by Orthopaedic Surgeons and Trainees in Australia
J. Churchill
016 Accuracy of Mobile Phone Pedometer Technology
G Boyce, G Padmasekara, M Blum
023 Accuracy of using a tablet device for the use of digital radiology manipulation and measurements
Y Edirisinghe, M Crossette
Case Reports
028 Video Multimedia Messaging System (MMS) supporting referral for an acute subdural haemorrhage: A case report
K Scandrett
In keeping with our open-access principles, all articles are published both as full text and as PDF files for download. For your convenience, attached to this post is a PDF file containing the complete Volume 1, Issue 2, which can be easily downloaded and saved for viewing offline.
We look forward to hearing from readers in the comments section, and encourage authors to submit research to be considered for publication in this peer-reviewed medical journal.
Yours Sincerely,
Editorial Board
Journal of Mobile Technology in Medicine
Posted on Jun 1, 2012 in Articles, Editorial |
Dr Orrin Franko MD2
1Lead App Editor, Journal of Mobile Technology in Medicine, 2Dept of Orthopaedic Surgery, University of California, USA.
Corresponding Author: ofranko@ucsd.edu
Journal MTM 1:2:1-4, 2012
doi:10.7309/jmtm.9
The last two years have demonstrated an exponential growth in the use of smartphones and tablets by medical professionals, a trend that has led to medical apps developed specifically for patients and physicians.1-71. Azark R. Smartphone apps for your practice. CDS Rev 2011;104:12-13.
2. Bhansali R, Armstrong J. Smartphone applications for pediatric anesthesia. Paediatr Anaesth 2012;22:400-404.
3. Franko OI. Smartphone apps for orthopaedic surgeons. Clin Orthop Relat Res 2011;469:2042-2048.
4. Franko OI, Bhola S. iPad apps for orthopedic surgeons. Orthopedics 2011;34:978-981.
5. Oehler RL, Smith K, Toney JF. Infectious diseases resources for the iPhone. Clin Infect Dis 2010;50:1268-1274.
6. Rosser BA, Eccleston C. Smartphone applications for pain management. J Telemed Telecare 2011;17:308-312.
7. Franko OI, Tirrell TF. Smartphone App Use Among Medical Providers in ACGME Training Programs. J Med Syst 2011. Not surprisingly, because most app developers are unverified sources of medical information, recent publications have emphasized the importance of peer-review validation.7-107. Franko OI, Tirrell TF. Smartphone App Use Among Medical Providers in ACGME Training Programs. J Med Syst 2011.
8. Boulos MN, Wheeler S, Tavares C, Jones R. How smartphones are changing the face of mobile and participatory healthcare: an overview, with example from eCAALYX. Biomed Eng Online 2011;10:24.
9. Hamilton AD, Brady RR. Medical Professional Involvement in Smartphone Apps in Dermatology. Br J Dermatol 2012.
10. Kabachinski J. Mobile medical apps changing healthcare technology. Biomed Instrum Technol 2011;45:482-486. In addition to safety concerns, the validation of mobile apps in the health care setting provides an opportunity for younger physicians, often medical students and residents, to contribute to the medical community by demonstrating the efficacy and validity of these new technologies. However, many trainees and practicing physicians are unfamiliar with scientific validation methodology. This editorial outlines a structure that can be used to assist with the design, execution, and publication of a validation study for mobile technology.
Validation refers to proving a tool’s ability to report the absolute “truth” as much as it can be measured. Various forms of validity exist that, when combined, allow a tool to be considered “valid” by the medical community. To clarify various forms of validation, I will share examples from the current literature, which can serve as guides for providers interested in designing a study of their own.