Mind the gap – a study on mHealth based treatment process optimization in addiction medicine
Ulf Gerhardt1, Thomas Gerlitzki2, Ruediger Breitschwerdt3, Oliver Thomas1
1Information Management & Information Systems, Osnabrueck University, Germany
2Universitätsklinikum Hamburg-Eppendorf, Hamburg University, Germany
3Wilhelm Büchner Hochschule – Mobile University of Technology, Faculty of Informatics, Darmstadt, Germany
Corresponding Author: ulfgerhardt@uni-osnabrueck.de
Background: In rural areas, a considerable lack of therapy and self-help facilities has been described with regard to drug dependence treatment. Mobile technologies are supposed to bridge geographical distances and improve access to healthcare.
Aim: The paper therefore aims to compare conventional vs. mHealth supported delivery processes in drug dependence treatment.
Methods: We use BPMN process modelling to compare usual vs. mHealth assisted treatment pathways for drug addiction. The details of mHealth support (mHealth configuration, monitoring, interventions, information processing) are also demonstrated.
Results: The paper shows (1) that the medical treatment gap mainly occurs at the interface between inpatient and outpatient care and (2) that mHealth support eliminates this interface problem. mHealth effectively supports drug dependence treatment in rural areas.
Conclusion: The paper demonstrates an mHealth based optimization of a complex treatment process. Our approach is also expected to improve theoretical and practical knowledge in mHealth service engineering.
Keywords: patient-therapist collaboration, mHealth, clinical pathway, drug addiction, BPMN, interface
Introduction
IT for healthcare professionals
Performance and service quality of healthcare crucially depend on the availability of information1. The high complexity of medical processes also meets IT based support at the point-of-care2. Therefore, modern mobile IT applications have the potential to assist with improving healthcare outcomes and adapting the treatment as appropriate for the current situation. In this regard, particular attention has been paid to the healthcare perspective: hospital staff, practitioners and therapists, community nursing and paramedic staff or rescue workers have been addressed as target groups3,4,5,1.
The patient perspective
Improved use of information fosters not only professional orientation for providers but also their patient relationship6,7. Therefore, some working groups8,9 have already dealt with provider-client-interaction, i.e. (tele-)consultations. On the consumer or patient side, this could also be facilitated by long-established mobile IT usage, particularly of smartphones and corresponding applications10,11,12.
This contribution uses a broad perspective including affected patients, professional healthcare providers, from the field of psychiatry and psychotherapy, and necessary treatment pathways. On the one hand, as described in detail in Chapter 4, the conventional addiction treatment process contains serious gaps13,14, in particular due to the physical distance of drug- dependent outpatients from specialized healthcare providers. On the other hand, Tryon et al.15 emphasize patient–therapist collaboration to enhance psychotherapeutic outcomes. Following Oates16 (pp. 296–298) for a critical research perspective, this contribution challenges the status quo but could also help with fundamentals for empowering patients. This results not only in maintaining a more seamless care but may also leads to improved shared decision making, patient-centricity and better autonomy and independence from health service providers. It might also assist with patient collaboration in terms of electronic availability of self-help groups. This also corresponds to Probert’s17 (pp. 137, 151) call for linking social aspects, such as relationships, of stakeholders with meeting organizational or economic pressures (e.g. costs) while providing users more control over the IT applications.
Motivation and introduction to the research problem
One author of the present study is a senior physician in a counselling centre specializing in addiction diseases treatment. In his more than 10 years’ experience, drug-dependency disorders are chronic diseases with patterns of complex mental, physical and socio economic damage. In contrast to ‘purely’ alcohol-dependent patients, the abstinence rate of drug-dependent individuals is much lower, partially attributable to a considerable lack of drug-specific therapy and self-help facilities, especially in rural areas13,14,18.
To bridge this gap, the German Centre for Addiction Issues (german: Deutsche Hauptstelle für Suchtfragen / DHS) demands the development of digital solutions to complement and optimize the existing analogue therapy processes14. It has already been proven that mobile technologies in particular are suitable to compensate for the greater geographical distances and the resulting inefficiency and supply deficits in rural areas19,1. A recent study with participation of key stakeholders created the research hypothesis that “a mobile technology- based system could (a) be successfully implemented for patients recovering from drug dependence and (b) improve the outcome for these patients”18.
This resulted in the motivation to systematically examine the treatment process for drug dependent diseases with regard to the optimization potential through mHealth systems. In the present study, this could be achieved by close cooperation between healthcare providers and two academic IS research institutions (Information Management & Information Systems, Osnabrueck University, Germany; Faculty of Informatics, Wilhelm Büchner Hochschule – Mobile University of Technology, Germany).
Research objective
The study aims to evaluate the scientific hypothesis that an mHealth application might be suitable for process optimization in the area of drug dependence treatment. Our Business Process Model and Notation (BPMN) based approach of comparing (conventional vs. mHealth supported) delivery processes is also expected to provide theoretical and practical knowledge that might be useful with regard to mHealth service engineering.
Method
In contrast to natural sciences (aiming to map the status quo as precisely as possible), problem solving and further development of complex systems require the detection and evaluation of novel, i.e. not yet existing approaches. In this context, typically more than one single solution can be regarded as effective or “correct”. The number of possible solutions may also increase over time, so that additional solutions may come possible due to changed theoretical frameworks or due to new technological or social conditions. These solutions are called “artefact” if they “have, or can be transformed into, a material existence as an artificially made object (e.g., model, instantiation) or process (e.g., method, software)”20. For innovative (socio-)technological objectives, such as the one described above, design-oriented approaches are proposed for healthcare: for modelling21 and for the use of healthcare IT22.
In the initial phase of Design Science (DS), purely intuitive procedures dominated the handling of artefacts. In order to convert this randomized “hacking”23 into a verifiable, targeted scientific process, an appropriate research methodology is essential.
From 2004 to date, based on the seminal works by Simon24, Hevner25,20,26 and Peffers27,28 essential milestones of DS Research Methodology (DSRM) including 6 consecutive research “activities“ have been developed and published. The basic DSRM reference work by Peffers28 already pointed out “there is no expectation that researchers would always proceed in sequential order from activity 1 through activity 6. In reality, they may actually start at any step and move outward”.
As the present research project arises from a “problem centred initiation”28 (p. 54, figure 1), it starts with DSRM activity 1 (problem identification & motivation) and extends to activity 2 (definition of the objectives for a solution) in the following28 (pp. 52–56). According to the “DSR knowledge contribution framework”, our main scientific contribution is an “improvement”, as the solution maturity is low (i.e. a new solution is studied) while the application domain maturity is high (i.e. a known problem is studied).
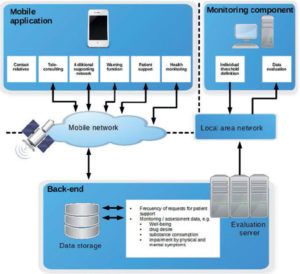
Figure 1: Architecture of the solution from51, refined and updated
For best-possible orientation within the present paper, Table 1 correlates the DSRM activities components to the corresponding chapters.

Table 1: DSRM activities of the present research paper (in accordance with Peffers28 pp. 52–56)
We are convinced that the understanding and mHealth based optimization of a complex treatment process can be strongly supported by suitable visualization and representation in models; such reference representations may also increase process and outcome quality29.
Another advantage of this approach could be an increased healthcare transparency for patients and their relatives30. Standardized health care processes are also considered essential for patient safety31. For psychiatry, it has previously been undertaken to model care pathways32 (for definition of clinical pathways see Table 2).
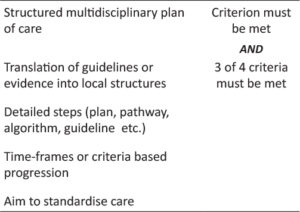
Table 2: Definition of clinical pathways in accordance to Aspland43 and Kinsman44
The BPMN standard is well-established in clinical IS development33. The corresponding methodology has been developed by the Business Process Management Initiative (BPMI, later merged with the Object Management Group OMG) to ensure comprehensible presentation, monitoring and control of complex processes. through the years34, BPMN has become “the de facto and ISO (International Standards Organization, Geneva) standard for process modelling, providing support for modelling control flow, data flow and resource allocation”. It also allows for explicit modelling of the collaboration between various stakeholders by use of so-called swimlanes (cp. Fig. A4, for instance). BPMN has been proven to be easily understood by stakeholders, to represent the real organizational processes and to facilitate a translation of business models into business process execution language35. Pryss et al.2,36 successfully used BPMN for process conceptualization and realization on mobile devices (2011) and surveyed its usage for clinical process visualization as helpful (2015).
The paper at hand therefore uses BPMN process modelling to investigate to what extent a drug-specific mHealth application offers potential for reducing barriers in the processes of drug addiction treatment and aftercare with the main intention to close the shortage of care in sparsely populated regions.
Introduction to the medical challenge
In Germany, about 1.7 million people suffer from alcohol addiction, and worldwide 3.3 million deaths result from alcohol related disease every year37. Dependency disorders lead to poverty and numerous secondary diseases such as liver cirrhosis, polyneuropathy, psychosis, seizure, persistent personality changes and chronic infectious diseases. In the US, recently a national emergency was declared due to the increased deaths associated with opiate dependence38.
As alcohol is the main addictive substance, the corresponding German medical care structures for alcohol dependence are developed to a very high standard. These include addiction counselling centres, where patients receive low-threshold counselling, motivational therapy and – and after gaining sufficient intrinsic motivation – referral to the medical treatment. In Germany, the gold standard is considered to be an inpatient withdrawal treatment, ideally following a detoxification treatment to eliminate the addictive substance from the body under medical control of the risks (eg seizures or a potentially life-threatening delirium)39. Unfortunately, a definitive cure for an addictive disorder is not possible, so that a “maintenance therapy” is required after the weaning treatment. This includes an outpatient follow-up treatment of about 6–12 months carried out by addiction therapists and physicians in outpatient institutions or, again, in the addiction counselling centres. Lifelong participation in self-help groups has proven to be prophylactic against relapse40,41.
At first glance, these medical care structures are available for drug dependent patients as well as for alcoholics. However, the treatment structures for drug users show a clear geographic heterogeneity in contrast to a nationwide sufficient treatment for alcohol dependence. While there are sufficient drug-specific offers of follow-up care and self-help work in larger cities and agglomerations, there are neither enough addiction specialists in the sparsely populated German regions nor appropriate drug-specific addiction self-help groups. Only 2.5 % of drug dependent patients have access to adequate groups, so that the German Centre for Addiction Issues (DHS) names drug dependent persons as a “target group (that has) not (been) reached”13. Consequently, there is a gap in the drug addiction treatment process with regard to drug-specific therapy and self-help which probably contributes to the high relapse rate of about 90% within a year of discharge after inpatient detoxification treatment42.
Clinical pathways are used as “an effective and efficient approach in standardising the progression of treatment, to support patient care and facilitate clinical decision making” 43 as shown in Table 2.
Numerous healthcare process modeling publications focus on standardized clinical treatment pathways with rather selective, technical focus, e.g. optimization of operating theatre activities45 or radiation therapy treatment planning46.
However, the lack of resources in medicine makes it particularly necessary to focus also those complex chronic diseases causing relatively high resource consumption by repeatedly passing through different medical sectors and supply levels. It is obvious that the identification of possible ways out of this vicious circle is not only economically necessary, but can especially offer a significant quality of life improvement for those patients concerned.
Towards a solution/Introduction to mHealth
mHealth offers the possibility of providing information and medical services even in sparsely populated regions by using a sophisticated IT infrastructure. In a country with many remote areas, for example, an information platform has been established47 for midwives who would otherwise have had no access to up-to-date specialist information. Another typical example is an application supporting rural medical services48,49.
Considering telepsychiatric settings, there is evidence for sufficient efficacy “for psychiatric assessment and treatment in the adult, child, and geriatric populations”50. However, as yet there is no marketable mHealth product for the most affected group of drug dependent patients .On the other hand, concrete elements / requirements of such an application have been described in detail involving experts on the patient and therapist side18. Its basic feasibility has been shown51 meaning that it could “be transformed into a material existence as an artificially made process (e.g., software)” and therefore – strictly speaking – represents an “artefact” in the sense of DS20.
In accordance to51, we propose a possible architecture of the mHealth system:
Resultsi
Classical treatment pathway for alcoholism (Fig. A1)
After the admission procedure in the addiction clinic with specialist diagnosis and therapy plan preparation, the inpatient therapy process, i.e. the actual addiction therapy, begins. The core of this sub-process is a daily group (max. 12 patients) psychotherapy and accompanying individual therapy performed by inpatient addiction therapists. In addition, there is a broad spectrum of individualized therapy modules depending on the physical and emotional comorbidities of the patient. This can range from multimodal pain therapy to trauma-specific therapy offers and also concepts for professional reintegration. As one result of such a multi- faceted and profound inpatient treatment lasting on average 15 weeks, the inpatient addiction therapist usually obtains a very comprehensive picture of those patient- and context-related factors contributing to the development of the addiction disorder. In a second step, individual coping strategies can be developed. This comprehensive and differentiated addiction therapeutic information package will be further updated in the context of a cross-professional final conference and a final medical examination and then forwarded as standard by the inpatient addiction therapist to alcohol-specific aftercare providers.
To ensure a sufficient information flow, the hospital discharge report is preceded by a telephone conference. This ensures that (similar to e.g. an electrocardiogram [ECG] or magnetic resonance imaging [MRI] finding in internal medicine) at the interface between inpatient addiction treatment and aftercare, the information obtained during inpatient stay will be available seamlessly in the alcohol-specific follow-up treatment. As a result, the generally available outpatient therapists (specialized in the treatment of alcoholism) can directly refer to the existing individual disorder models, coping strategies and crisis intervention techniques.
Classical treatment pathway for drug addiction (Fig. A2)
The inpatient drug addiction treatment course from the admission procedure to the inpatient therapy process to the final case conference and final examination is comparable to that already described for alcoholism treatment. It generates as much post-stage essential information regarding individual disorder models, coping strategies and crisis intervention techniques. Of course, a seamless further therapeutic use of this differentiated support package would be highly desirable and useful especially for the young and socioeconomically as well as medically severely impaired drug dependent persons.
Unfortunately, there is often neither a drug-specific follow-up treatment with appropriately qualified outpatient addiction therapists available. This also concerns drug-specific self-help groups for the large number of drug dependent patients living in the sparsely populated regions outside the major cities and metropolitan areas.
A comparison of the illustrated drug addiction specific treatment pathway with that for alcohol dependency clarifies the termination of both the process flow and the flow of information at the interface between the inpatient drug addiction treatment and the outpatient follow-up treatment. Exactly this phenomenon is also felt by the patients concerned18: they are cut off from further drug-specific treatment or self-help. That means that the solutions developed in the intensive inpatient treatment cannot be used efficiently, but “sand down” due to a lack of drug-specific outpatient support.
The likelihood of re-developing a desire to consume drugs due to post-hospital personal and occupational stressors, and then to relapse due to the loss of those coping strategies developed during inpatient therapy, is obvious.
The resulting extreme high relapse rates threaten the individual patient’s health, but also represent a huge economic burden: e.g., as increased costs of crimes by drug-using persons, as lower social security contributions or as growing numbers of high-frequency acute hospital treatments (e.g., acute detoxification, treatment of expensive addictive consequential damages).
mHealth assisted treatment pathway for drug addiction (Fig. A3)
Compared to the above-mentioned classical treatment pathway for drug addiction, the inpatient addiction therapists provide training for the patients with regard to the future implementation of a mHealth application during the actual inpatient therapy. The developed individual disorder models, coping strategies and crisis intervention techniques are then used for the individualized configuration of a mHealth application in parallel to the inpatient therapy process.
The mHealth application also offers the possibility to store the detailed information about the inpatient therapy together with resulting therapy recommendations and coping strategies in password-protected form (see section 7c: mHealth information processing). This is because a direct transfer of information between inpatient addiction therapists and (because non-existent) drug-treatment-specialized follow-up therapists is not possible. At discharge, a multi- dimensional mHealth support can begin.
The mHealth application regularly monitors important mental parameters such as depressivity, drug desire and drug relapse. The results are processed via a server and viewed by an inpatient addiction therapist who in turn has various intervention options (see section 7b: health monitoring and mHealth interventions).
In addition, the mHealth application itself offers several options to counteract adverse events such as an impending relapse: A low-threshold opportunity to contact with important caregivers, a chance to meet with other stakeholders in the sense of a spontaneously formed self-help group and the possibility of direct patient support through the mHealth application. The corresponding key caregivers and successful strategies for mHealth-based patient support (e.g., use of relaxation procedures, memory of important therapeutic goals, physical exercise, etc.) have been previously been set up as part of the inpatient app configuration. Furthermore, the mHealth application offers the possibility to activate a location-based warning function using the smartphone’s GPS system, i.e. alerting the drug-addicted user if approaching one of his former typical drug-buying and consuming places. The corresponding location information has already been implemented in parallel to the inpatient therapy process.
Two special features should be emphasized: first, there is an input option for the addiction therapists so that – in the absence of well-informed outpatient follow-up therapists – the general and individual expertise of drug-specialized addiction therapists in inpatient settings will be used.
Second, the mHealth application may provide the appropriate physicians and therapists with the high-detail discharge report package if the patient is to receive further medical and / or therapeutic treatment in the future. This includes individual crisis intervention and coping strategies generated during inpatient addiction treatment, thus significantly increasing outpatient treatment efficiency. In order to protect these particularly sensitive data from unauthorized access, they are encrypted on the one hand and not stored on the patient’s end device on the other hand (see section 6f: mHealth information processing).
Individualized configuration (Fig. A4)
During the inpatient stay, training of the future app users is conducted by a trained inpatient addiction therapist (“trainer”) in parallel to the therapy process. As part of this structured training course, the trainer first provides information on the installation and activation of the mHealth application. After successful activation, the trainer educates the future users in relation to the functions contained in the application. At the same time, the application carries out a self-configuration and guides the user through a structured information recording. Contents are the user profile, important caregivers as well as motivational aids developed in the inpatient therapeutic course of treatment (including individual disorder models, coping strategies and crisis intervention techniques).
The more complex alerting function is part of the training. Those users willing to set up this function are guided by the app through a suitable implementation module. Past purchase and consumption locations are individually maintained and the alerting function is set up correspondingly. At the end of the app setup and customization process, the app functionality is tested (for technical implementation see section 6g: synergism of IT and therapists in mHealth individualization).
Health monitoring & mHealth interventions (Fig. A5)
The monitoring is initially based on a structured exchange of information between the user and the mHealth application. As a result, the monitoring / assessment data such as e.g. well- being, drug desire, substance consumption and subjectively perceived impairment by physical and mental symptoms are transferred to the evaluation server under strict observation of data security regulations. On the basis of predefined scores, the evaluation server is able to prioritize the raw data from monitoring and, in the event of impending decompensation (“high risk”), sends a corresponding alert message to the inpatient addiction therapist responsible for intervention decisions. Thus the latter is well informed about the corresponding intervention priority.
After reviewing the patient profiles, the inpatient addiction therapist has the option either to contact the patient directly and to give individual assistance or, if available, to include a non-specialized follow-up caregiver (for example family doctor or psychotherapist) after reviewing the patient profiles. However, he or she may also propose an intervention selectable from a portfolio of suitable measures formerly prepared during the inpatient app customization.
mHealth-based information processing (Fig. A6)
The highly informative mHealth package of discharge report plus individual crisis intervention and coping strategies generated during inpatient withdrawal treatment may be very helpful for sufficient outpatient treatment, but it also contains highly sensitive data. Therefore, a process-oriented presentation of the mHealth application should also include information about the mHealth information sharing process.
If both the inpatient addiction institution and non-specialized aftercare providers use the same information (e.g. from EPA-S medical record via a hospital information system such as PATFAK, a domain specific HIS) reliably evaluated with regard to security, the mHealth-based information processing is relatively easy given the corresponding consent of the patient. However, if the data transfer is to take place outside of such a protected system, it must be ensured that the patient remains “master of his data”. For this purpose, in the illustrated mHealth process BPMN model we propose that an encrypted file is created and sent to the non-specialized aftercare practitioner upon request by the patient via the mHealth app.
The password required to open and decrypt the files is submitted exclusively to the patient who then has complete control over the processing of his data. If he or she wants to make the information available to a caregiver, he or she can forward the password either by phone or alternatively through personal contact.
Synergism of IT and therapists in mHealth individualization (Fig. A7)
The therapeutically moderated individual customization of the mHealth application as described must also be technically represented. As with other IT solutions in healthcare, this is done by the application specialists in the IT department of the respective inpatient addiction clinic.
After hospitalization of a patient with substance use disorder, these application specialists can create a link between the newly created patient profile and the account of the responsible reference therapist in the hospital information system. The therapist who is automatically informed of this action has the opportunity to prepare information from the therapeutic progress report and the discharge report. She or he can also organize the results of the case conference at the end of inpatient treatment in such a way that they can be deposited in compliance with data protection regulations.
The information can be made available to other caregivers at a later stage, but only if the patient has consented (see Section 6f: mHealth information processing). In addition, a patient- individual adjustment of the application is performed by the responsible therapist during the inpatient stay. The inpatient therapist also checks to what extent the configuration steps controlled by the application itself have been successfully completed (e.g., the registration of supporting persons).
In addition, support measures already set by the patient (e.g., with regard to individual disorder models or coping strategies, e.g. stress management, deflection, relaxation techniques) may be modified by the reference therapist. In practical implementation, this would presumably mean that the reference therapist and the patient jointly work on these mHealth individualization aspects particularly important for the success of the treatment – or at least intensively discuss these aspects. In order to enable monitoring and targeted / controlled intervention options after discharge from inpatient treatment, the reference therapist also sets individual threshold scores. These relate to important addiction-related medical parameters such as well-being, drug desire, substance consumption and individually perceived impairment of physical and emotional symptoms.
Evaluation & Discussion
In general, any mHealth initiative requires rigorous evaluation52 which is in line with the chosen design science approach. Inherent to design science, however, a thorough evaluation before implementation should be considered as debatable since their effects cannot, from a logical perspective, already be part of the development. An evaluation also depends on the ultimate individual benefit in practice. This can therefore be analysed satisfactorily – if at all (e.g. Schryen53 doubts whether the added value of information systems in organizations can be rigorously demonstrated) – only ex post. That analysis requires real-life experiences with applications and the resources required for design science projects54.
Critical research also denies the possibility of such objective knowledge55 as often suggested by DS evaluations. Therefore, at this point we primarily evaluate in a descriptive manner as legitimized by relevant scholars25. Initial patient interview results advocated moving forward from the status quo18, based on the evidence for sustainable mHealth engineering56 and mHealth technology57. As a result, the concept and base architecture as updated in Figure 1 and the overall approach51 have been welcomed by scholars at a German information systems conference.
So far, reviews on the topic of digitalization in healthcare have focused mainly on the inpatient area58. But patient satisfaction after hospital treatment closely depends on interface communication (between the inpatient and outpatient sector)59. First, prior contributions let expect lowered barriers in relationships to the providers due to mHealth, and secondly positive outcomes on telepsychiatry in general60 as well as for addiction scenarios in particular61.
As added value, the facilitated healthcare delivery should be highlighted since it benefits patients, service providers, and other stakeholders in this context. It is also considered a paradigmatic goal of IT support for the domain62. The use of IT leads to more confidence, empowerment, increasing knowledge and inproved health status, while neither a lack of face- to-face meetings nor a lack of privacy appeared problematic to stakeholders. Partly, they evaluated it better than face-to-face settings, to be more private, intimate or comfortable6,8. Amongst consumers, there is openness to accept mHealth support63,49, and not only patients but also professionals confirm the quality of joint therapeutic alliance in telepsychiatry as to be adequate to face-to-face64.
A recent literature review65 supports the use of BPMN as “an effective methodology to optimize clinical processes” and pointed out that it “has proven to be a feasible and useful methodology to design and optimize clinical processes, as well as to automate tasks”. Weber34 showed that BPMN can also be used to identify conflicts between clinical care guidelines. The OMG Healthcare Domain Taskforce denotes BPMN as “our choice for workflows” in clinical pathway modeling66.
The comparison between the classical treatment pathways shows a striking difference between the supply processes of alcohol dependency versus drug addiction outside metropolitan areas. This medical treatment gap does not manifest during inpatient addiction treatment, but rather at the interface between inpatient and outpatient care. The presentation of an mHealth-supported treatment pathway shows that an mHealth application is suitable for alleviating or eliminating precisely this interface problem. This results in an effective support for these often young and medically and socioeconomically severely affected patients.
Our approach has not been necessarily targeted to minimize or reduce treatment steps, but to fill today’s gaps of care. Comparative modelling of current and mHealth treatment pathways shows that the latter meets the requirements for bridging such supply gaps as recently identified by an approach including structured expert interviews18,51:
Control: on the one hand, the mHealth application regularly collects treatment-related parameters such as well-being, drug desire, substance consumption and subjectively perceived impairment by physical and mental symptoms. Due to cut-off values defined during the inpatient stay, the data collected can be automatically prioritized and, in urgent cases, rapid assistance can be offered. On the other hand, the application also warns patients against geographical proximity to places where they preferred to consume drugs before their inpatient therapy or where they have bought their addictive substances. This is empowering patients and particularly significant from the critical research background.
Relationship: the establishment of a stable relationship can be achieved regardless of the medium, with Internet-based forms of therapy being assessed by both patients and therapists comparable to conventional face-to-face therapies64. The mHealth application contains a contact feature that allows a patient to contact her or his important caregivers at any time for teleconsultation as advocated by Åkesson6, but especially in critical situations. Important collaborators may include both private (e.g., parents, siblings, spouse) and professional (e.g., family doctor, legal guardian, physicians, and therapists) supporters. Those important caregivers are individually identified during the inpatient treatment in close cooperation with the inpatient´s reference therapist. In addition, the application contains another function, which is to enable the formation of an additional supporting network. This function had been proposed by the patients themselves as a result of participative requirement engineering18. It uses the smartphone GPS system for identification of geographically nearby like-minded people and the opportunity to establish a kind of “spontaneous self-help group” and to stabilize each other.
Therapeutically oriented patient support: in addition to the monitoring and contact- enabling components, the application also contains therapeutic functions in the narrow sense. This is achieved through the targeted, individualized use of the individual disorder models, coping strategies and crisis intervention techniques developed during inpatient therapy and also by the application-based use of standardized therapeutic relaxation methods. In addition, if the evaluation server has identified impending decompensation (“high risk”), the responsible inpatient addiction therapist may in turn propose a supportive intervention to the patient.
Storage and disseminating of therapy-relevant information: an important therapeutic issue is to document the psychotherapy progress and to pass on important therapeutic findings to the following outpatient specialist as promptly and seamlessly as possible. This interface between inpatient addiction therapy and outpatient treatment follow-up is precisely where the supply gap has persisted. For this reason, and as previously pointed out, at the time of transition between the inpatient and outpatient sectors it is often unclear whether further drug-specific follow-up treatment will be possible at all and who will carry out this treatment.
Thus, the mHealth application contains a special function for the backup and data protection-safe transfer of therapy-relevant information. Together with the hospital discharge report and the result of the cross-professional final case discussion, the individually developed disease models and intervention options are stored in a password-protected document. This document can only be reopened with patient consent.
If the patient moves to an area with adequate follow-up treatment or undergoes a new inpatient weaning treatment, the suggested mHealth solution allows the drug-specialized addiction therapists to adopt and continue where inpatient therapy had ended. However, it can also be used for further caregivers even if non-drug- specifically trained, e.g. general practitioners, psychotherapists, acute hospitals or addiction counselling centres specialized in alcohol addiction treatment. For both specialized and non-specialized caregivers, the application is an extremely valuable source and is provided in a patient-centric way.
Restrictions: the individualized mHealth application can basically only work if the appropriate individualization parameters are identified and implemented into the application during inpatient addiction treatment.
In addition, the best-possible functioning of the application depends on appropriate staff resources being available in the inpatient clinics for the individualization of the application, for the evaluation of the data transmitted by the application and for therapeutic interventions.
Conclusion & Outlook
The paper at hand offers evidence that BPMN represents the standard for process modelling not only in economic contexts34,35, but also to optimize clinical processes65,34. Furthermore, in comparison to the classical treatment pathway, individualized mHealth-support eliminates the interface problem from occurring between the various treatment providers and thereby bridges the existing supply gap18.
A challenge could be to refine a viable business model of the mHealth application that can roughly be describe as based on the Business Model Canvas approach67 and its nine dimensions: value proposition; supply side with key partners, activities and resources; customer side with channels, customer segments and relationships; financial side with cost and revenue structure. As a value proposition, the mHealth app will be able to close the treatment gap occurring at the interfaces between inpatient and outpatient segments of drug addiction treatment. Besides therapy-relevant information storage, on a patient-to-patient basis in virtual self-help groups, the main mHealth related service improvements in addiction treatment refer to patient support, better control of the treatment process, an enhanced relationship between patient and caregivers or more barrier- free collaboration.
Before refining supply, customer and finally financial aspects of the business model canvas, five mHealth domain-specifics – ethical and sociocultural aspects, data protection, technology and location-independence – should be further considered68.
As discussed against the background of John Rawls’ theories69, from an ethics perspective, any patient or healthcare delivery support has to be welcomed, as long as fair and equal access can be granted counteracting the digital divide. Following proposed guidance such as the European Code of Practice for Telehealth Services70 or the codes listed by ZUR Institute71 should ensure ethical compliance. The sociocultural conception must address cost-sensitivity of healthcare systems72, in particular organizations responsible for reimbursement (such as in Germany’s health system), its professional providers73 and – again for counteracting the digital divide – consumers (cp. also financial side at the end of this section). Also, adoption issues74 require measures such as an action plan for successful and continued usage (as for any organizational application). This includes educational resources, updates and, as scholars suggest for organizations integrating telemedicine or consultation support75,76. The advantages of teleconsultation such as fewer medical errors77 or better access could generate rising acceptance by consumers thus contributing to their usage motivation. The Shamrock model provides further critical success factors for service deployment75.
In regard to data protection, and supporting Peddle’s requirements73, national (in Germany, state and for confessional hospitals also church laws can apply) and European Regulation (especially GDPR) have to be followed: this also fostering trust, another sociocultural aspect. It can be realized by encryption technology, safe user accounts and the client’s confirmation or self- definition of privacy preferences. However, a completely anonymized service would not work between therapist and client. Technology-wise, platform and hardware independence of clients’ devices must be guaranteed. Use of wide-spread syntactic standards in the domain – such as HL7 and its derivate Fast Healthcare Interoperability Resources (FHIR) for communication with mobile devices – is recommended. As specified earlier, the application should run location-independently: especially outside of clinics.
Referring back to the Business Model Canvas, customers in terms of main beneficiaries in our understanding will represent patients with a drug addiction (e.g., ICD-10 code: F19.2) without any social, demographic or geographic restriction. The main distribution channel may be a recommendation from the treating addiction clinic staff; in addition, also distribution via social networks or word-of-mouth is possible. On the one hand, keeping and growing customer relationships could be achieved directly within the app and on the other hand by contacting the client electronically.
Key resources in development will be of financial (e.g. insurance companies, public grants, software companies) and intellectual kind (medical knowledge, software engineering, programming) as well as physical resources (servers, mobile devices). Key partners of such a project may be insurance companies, public sponsors, software companies, research facilities and drug addiction healthcare providers. Key activities, after successfully implementing the mHealth app in the market, will be updating the therapeutic monitoring and intervention aiming at closing the treatment gap and improving the outcome in drug dependency. Main mHealth related improvements in addiction treatment refer to control of the treatment process, relationship between patient and caregivers, patient support, and therapy-relevant information storage.
If the app is approved as a medical device or accepted in terms of options abiding to recent DiGAV-act78, the revenue model can be based on health insurance benefits in Germany. This seems to be necessary, as from experience a large portion of the clients are economically not capable to independently fund costs (on provider side: staff, software, hardware). At this point, a cost calculation from a provider perspective has not yet been fully completed. We do estimate that the mHealth app development and implementation represent rather minor costs compared to the main share stemming from long-term therapeutic mHealth care.
Future work in this field will have to focus on transforming our artefact into “material existence”20, i.e., a marketable mHealth app with the aim of clinical efficacy studies and finally official approval. An alternative model could only be realized for a customer group both willing and able to self-pay. Since cost-effectiveness of telemedical solutions has been proven79, expected benefits or outcomes should outweigh any costs easily when the solution is thoroughly implemented.
References
1. Breitschwerdt, Rüdiger; Philipp Reinke; Markus Kleine Sextro; and Oliver Thomas (2012). Process-oriented application systems for mobile healthcare services: empirical analysis of professional users’ intention. ACM SIGHIT Record, vol. 2, no. 2, pp. 22–30.
2. Pryss R, Tiedeken J, Kreher U, Reichert M. Towards Flexible Process Support on Mobile Devices. In: Soffer P, Proper E, editors. Information Systems Evolution: 6th CAiSE Forum 2010 Selected Extended Papers (22nd CAiSE conference). Berlin, Heidelberg: Springer; 2011. p. 150–165. doi:10.1007/978-3-642-17722-4_11.
3. Chatterjee S, Chakraborty S, Sarker S, Sarker S, Lau FY. Examining the success factors for mobile work in healthcare: A deductive study. Decis Support Syst (Decision Support Systems). 2009;46:620–33. doi:10.1016/j.dss.2008.11.003.
4. Dünnebeil S, Sunyaev A, Blohm I, Leimeister JM, Krcmar H. Determinants of physicians’ technology acceptance for e-health in ambulatory care. Int J Med Inform (International Journal of Medical Informatics). 2012;81:746–60. doi:10.1016/j.ijmedinf.2012.02.002.
5. Kijl B, Nieuwenhuis B. Deploying a Telerehabilitation Service Innovation: An Early Stage Business Model Engineering Approach. In: IEEE, editor. New York: IEEE; 2010. p. 1–10. doi:10.1109/HICSS.2010.134.
6. Åkesson KM, Saveman B-I, Nilsson G. Health care consumers’ experiences of information communication technology – A summary of literature. Int J Med Inform (International Journal of Medical Informatics). 2007;76:633–45. doi:10.1016/j.ijmedinf.2006.07.001.
7. van Gurp J, van Selm M, van Leeuwen E, Vissers K, Hasselaar J. Teleconsultation for integrated palliative care at home: A qualitative study. Palliative Med (Palliative Medicine). 2016;30:257–69. doi:10.1177/0269216315598068.
8. Islind AS, Snis UL, Lindroth T, Lundin J, Cerna K, Steineck G. The Virtual Clinic: Two-sided Affordances in Consultation Practice. Computer Supported Cooperative Work. 2019; 28, 435–468. doi: 10.1007/s10606-019-09350-3.
9. Fitzpatrick G, Ellingsen G. A Review of 25 Years of CSCW Research in Healthcare: Contributions, Challenges and Future Agendas. Computer Supported Cooperative Work. 2013; 22, 609–665. doi: 10.1007/s10606-012-9168-0.
10. Boulos M, Wheeler S, Tavares C, Jones R. How smartphones are changing the face of mobile and participatory healthcare: an overview, with example from eCAALYX. BioMed Eng OnLine. 2011;10:24. doi:10.1186/1475-925X-10-24.
11. Bert F, Giacometti M, Gualano MR, Siliquini R. Smartphones and Health Promotion: A Review of the Evidence. J Med Syst. 2014;38:9995. doi:10.1007/s10916-013-9995-7.
12. Luxton DD, McCann RA, Bush NE, Mishkind MC, Reger GM. mHealth for mental health: Integrating smartphone technology in behavioral healthcare. Professional Psychology: Research and Practice. 2011;42:505–12. doi:10.1037/a0024485.
13. Deutsche Hauptstelle für Suchtfragen (DHS) (2006) Sucht-Selbsthilfe: Beratung und Begleitung von suchtkranken Menschen und ihren Angehörigen im Internet. https://www.suchthilfesachsen.de/fileadmin/Dokumente/Allgemeines/Suchtselbsthilfemanual.pdf (Accessed 05 November 2020).
14. Deutsche Hauptstelle für Suchtfragen (DHS) (2019) Die Versorgung von Menschen mit Suchtproblemen in Deutschland, Update 2019. https://www.dhs.de/fileadmin/user_upload/pdf/dhsstellungnahmen/Die_Versorgung_Su chtkranker_in_Deutschland_Update_2019.pdf (Accessed 05 November 2020).
15. Tryon GS, Birch SE, Verkuilen J. Meta-analyses of the relation of goal consensus and collaboration to psychotherapy outcome. Psychotherapy. 2018; 55(4): 372–383. doi: 10.1037/pst0000170.
16. Oates BJ. Researching Information Systems and Computing. London: Sage; 2005.
17. Probert SK. Adorno: A Critical Theory for IS Research. In J. Mingers and L. Willcocks (eds): Social Theory and Philosophy for Information Systems. Wiley Series in Information Systems. Chichester/ NJ: Wiley, pp. 129–156. 2004.
18. Gerhardt U, Breitschwerdt R, Thomas O. Relapse prevention in drug addiction: addressing a messy problem by IS Action Research. AI and Society. 2015;30 (1):31-43. doi: 10.1007/s00146-014-0544-9.
19. Wälivaara B-M, Andersson S, Axelsson K. General practitioners’ reasoning about using mobile distance-spanning technology in home care and in nursing home care. Scand J Caring Sci (Scandinavian Journal of Caring Sciences). 2011;25:117–25. doi:10.1111/j.1471-6712.2010.00800.x.
20. Gregor SD, Hevner AR. Positioning and Presenting Design Science Research for Maximum Impact. MIS Quart (Management Information Systems Quarterly). 2013;37:337–55. doi:10.25300/MISQ/2013/37.2.01.
21. Görlitz R, Rashid A. Stroke management as a service – a distributed and mobile architecture for post-acute stroke management. In: AIS, editor. Proceedings 20th European Conference on Information Systems (ECIS) 2012: Barcelona, June 10-13. Atlanta: AIS; 2012. p. 107.
22. Hegde V, Raheja D. Design for Reliability in Medical Devices. In: IEEE, editor. 39th Annual Reliability and Maintainability Symposium (RAMS), 2010, San José/CA 2010 January 25−28: Proceedings. Piscataway, NJ: IEEE; 2010. p. 1–6. doi:10.1109/RAMS.2010.5448077.
23. UTS Software Engineering. Design Science and Action Research: Engineering and IT for tomorrow. 2016. https://www.youtube.com/watch?v=yYa5g50MHaY. Accessed 18 Jan 2021.
24. Simon HA. The Sciences of the Artificial. 3rd ed. Cambridge, MA: MIT Press; 1996.
25. Hevner AR, March ST, Park J, Ram S. Design Science in Information Systems Research. MIS Quart (Management Information Systems Quarterly). 2004;28:75–105.
26. Hevner AR. Expanding the Impacts of Design Science Research: Keynote, 26 February 2014. Paderborn/ Germany; 2014.
27. Peffers K, Rothenberger M, Tuunanen T, Vaezi R. Design Science Research Evaluation. In: Peffers K, Rothenberger M, Kuechler B, editors. Design Science Research in Information Systems. Advances in Theory and Practice: 7th International Conference, DESRIST 2012, Las Vegas, NV, USA, May 14-15, 2012. Proceedings. Berlin, Heidelberg: Springer; 2012. p. 398–410. doi:10.1007/978-3-642-
29863-9_29.
28. Peffers K, Tuunanen T, Rothenberger MA, Chatterjee S. A Design Science Research Methodology for Information Systems Research. J Manage Inform Syst (Journal of Management Information Systems). 2007;24:45–77. doi:10.2753/MIS0742- 1222240302.
29. Schoenbaum SC, Gottlieb LK. Algorithm based improvement of clinical quality. Brit Med J (British Medical Journal). 1990;301:1374–6. doi:10.1136/bmj.301.6765.1374.
30. Kaiser K, Seyfang A, Miksch S. Identifying Treatment Activities for Modelling Computer-Interpretable Clinical Practice Guidelines. In: Riaño D, Teije At, Miksch S, Peleg M, editors. KR4HC 2010 – Knowledge representation for health-care: ECAI 2010 workshop KR4HC 2010, Lisbon, Portugal, August 17, 2010 ; revised selected papers; 2nd KR4HC. Berlin: Springer; 2011. p. 114–125.
31. Scharmer E-G, Siegel E. Fehlermöglichkeiten im Umgang mit Narkosegeräten und deren Vermeidung.: Ein Überblick. [Equipment failures in anesthesia and how to avoid them. An overview]. Anaesthesist. 1997;46:880–9. doi:10.1007/s001010050482.
32. Jun GT, Morrison C, O’Loughlin C, Clarkson PJ. Which Diagrams and When?: Health Workers’ Choice and Usage of Different Diagram Types for Service Improvement. In: Cox P, Plimmer B, Rodgers P, editors. Diagrammatic Representation and Inference: 7th International Conference, Diagrams 2012, Canterbury, UK, July 2-6, 2012. Proceedings. Berlin, Heidelberg: Springer; 2012. p. 340–342. doi:10.1007/978-3-642-31223-6_45.
33. Peleg M. The Role of Modeling in Clinical Information System Development Life Cycle. Methods Inf Med (Methods of Information in Medicine). 2011;50:7–10.
34. Weber P, Filho JBF, Bordbar B, Lee M, Litchfield I, Backman R. Automated conflict detection between medical care pathways. J Softw Evol Proc. 2018;30:e1898. doi:10.1002/smr.1898.
35. Geambaşu CV. BPMN vs. UML Activity Diagram for Business Process Modeling. Journal of Accounting and Management Information Systems. 2012;11:637–51.
36. Pryss R, Mundbrod N, Langer D, Reichert M. Supporting medical ward rounds through mobile task and process management. Inf Syst E-Bus Manage. 2015;13:107–46. doi:10.1007/s10257-014-0244-5.
37. WHO – World Health Organization (n.d.). Management of drug abuse. http://www.who.int/substance_abuse/facts/en/ (Accessed 06 November 2018).
38. McCarthy M. US declares opioid epidemic a “national emergency”. Brit Med J (British Medical Journal). 2017;358:j3881. doi:10.1136/bmj.j3881.
39. Kiefer F, Batra A. Screening, Diagnose und Behandlung alkoholbezogener Störungen. 2020. https://www.awmf.org/uploads/tx_szleitlinien/076-001l_S3-Screening-Diagnose-Behandlung-alkoholbezogene-Stoerungen_2021-02.pdf. Accessed 24 May 2021.
40. Emrick CD, Tonigan JS, Montgomery H, Little L. Alcoholics Anonymous: What is currently known? In: McCrady BS, Miller WR, editors. Research on Alcoholics Anonymous: Opportunities and alternatives. New York: Rutgers Center of Alcohol Studies. p. 41–76.
41. Humphreys K, Blodgett JC, Wagner TH. Estimating the efficacy of Alcoholics Anonymous without self-selection bias: an instrumental variables re-analysis of randomized clinical trials. Alcohol Clin Exp Res. 2014;38:2688–94. doi:10.1111/acer.12557.
42. Bailey GL, Herman DS, Stein MD. Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J Subst Abuse Treat (Journal of substance abuse treatment). 2013;45:302–5. doi:10.1016/j.jsat.2013.04.002.
43. Aspland E, Gartner D, Harper P. Clinical pathway modelling: a literature review. Health Syst. 2019:1–23. doi:10.1080/20476965.2019.1652547.
44. Kinsman L, Rotter T, James E, Snow P, Willis J. What is a clinical pathway? Development of a definition to inform the debate. BMC Med. 2010;8:31. doi:10.1186/1741-7015-8-31.
45. Barbagallo S, Corradi L, Ville Goyet J de, Iannucci M, Porro I, Rosso N, et al. Optimization and planning of operating theatre activities: an original definition of pathways and process modeling. BMC Med Inform Decis Mak. 2015;15:38. doi:10.1186/s12911-015-0161-7.
46. Babashov V, Aivas I, Begen MA, Cao JQ, Rodrigues G, D’Souza D, et al. Reducing Patient Waiting Times for Radiation Therapy and Improving the Treatment Planning Process: a Discrete-event Simulation Model (Radiation Treatment Planning). Clin Oncol (R Coll Radiol). 2017;29:385–91. doi:10.1016/j.clon.2017.01.039.
47. Niemöller C, Metzger D, Berkemeier L, Zobel B, Thomas O, Thomas V. Designing mHealth applications for developing countries. Proceedings 24th European Conference on Information Systems (ECIS). 2016; paper 149.
48. Lippman JM, Smith SNC, McMurry TL, Sutton ZG, Gunnell BS, Cote J, et al. Mobile Telestroke During Ambulance Transport Is Feasible in a Rural EMS Setting: The iTREAT Study. Telemed J E-Health (Telemedicine and e-Health). 2016;22:507–13. doi:10.1089/tmj.2015.0155.
49. Wälivaara B-M, Andersson S, Axelsson K. Views on technology among people in need of health care at home. Int J Circumpol Heal (International Journal of Circumpolar Health). 2009;68:158–69. doi:10.3402/ijch.v68i2.18326.
50. Gajaria A, Conn D, Madan R. Telepsychiatry: effectiveness and feasibility. SHTT. 2015:59–67. doi:10.2147/SHTT.S45702.
51. Gerhardt U, Hindermann V, Kiesow A. Von der Analyse zum Design: Entwicklung eines mHealth-Systems als individualisierte Behandlungsform zur Rückfallprophylaxe bei Drogenabhängigkeit. Tagungsband Multikonferenz Wirtschaftsinformatik (MKWI). 2016; 643-654.
52. Nilsen W, Kumar S, Shar A, Varoquiers C, Wiley T, Riley WT, et al. Advancing the Science of mHealth. J Heal Commun (Journal of Health Communication). 2012;17:5– 10. doi:10.1080/10810730.2012.677394.
53. Schryen G. Revisiting IS business value research: what we already know, what we still need to know, and how we can get there. Eur J Inform Syst (European Journal of Information Systems). 2013;22:139–69. doi:10.1057/ejis.2012.45.
54. Collins A, Joseph D, Bielaczyc K. Design Research: Theoretical and Methodological Issues. J Learn Sci (Journal of the Learning Sciences). 2004;13:15–42. doi:10.1207/s15327809jls1301_2.
55. McKay J, Marshall P. A Review of Design Science in Information Systems. ACIS Proceedings. 2005; 5.
56. Gerhardt U, Breitschwerdt R, Thomas O. Engineering sustainable mHealth: the role of Action Research. AI & Soc. 2017;32, 339–357. doi: 10.1007/s00146-015-0640-5.
57. Gerhardt U, Breitschwerdt R, Thomas O. mHealth engineering: a technology review. Journal of Information Technology Theory and Application. 2018;19 (3), 82–117.
58. Hufnagl C, Doctor E, Behrens L, Buck C, Eymann T. DIGITISATION ALONG THE PATIENT PATHWAY IN HOSPITALS. In: AIS, editor. Proceedings of the 27th European Conference on Information Systems ECIS 2019: Stockholm & Uppsala, Sweden, June 8-14. Atlanta: AIS; 2019. paper 193.
59. Adams DR, Flores A, Coltri A, Meltzer DO, Arora VM. A Missed Opportunity to Improve Patient Satisfaction? Patient Perceptions of Inpatient Communication With Their Primary Care Physician. Am J Med Qual (American journal of medical quality : the official journal of the American College of Medical Quality). 2016;31:568–76. doi:10.1177/1062860615593339.
60. Ekeland AG, Bowes A, Flottorp S. Methodologies for assessing telemedicine: A systematic review of reviews. Int J Med Inform (International Journal of Medical Informatics). 2012;81:1–11. doi:10.1016/j.ijmedinf.2011.10.009.
61. Fishkind AB, Cuyler RN, Shiekh MA, Snodgress M. Telepsychiatry and e-Mental Health. In: McQuistion HL, Sowers WE, Ranz JM, Feldman JM, editors. Handbook of Community Psychiatry. New York, NY: Springer; 2012. p. 125–140. doi:10.1007/978- 1-4614-3149-7_11.
62. Graham S, Estrin D, Horvitz E, Kohane I, Mynatt E, Sim I. Information Technology research challenges for healthcare. SIGHIT Rec (ACM SIGHIT Record). 2011;1:4–9. doi:10.1145/1971706.1971708.
63. Alagöz F, Ziefle M, Wilkowska W, Valdez AC. Openness to Accept Medical Technology – A Cultural View. In: Holzinger A, Simonic K-M, editors. Information Quality in e-Health 7th Conference of the Workgroup Human-Computer Interaction and Usability Engineering of the Austrian Computer Society, USAB 2011, Graz, Austria, November 25-26, 2011. Proceedings. Berlin, Heidelberg: Springer; 2011. p. 151–170. doi:10.1007/978-3-642-25364-5_14.
64. Klasen M, Knaevelsrud C, Böttche M. Die therapeutische Beziehung in internetbasierten Therapieverfahren : Ein Überblick: The therapeutic alliance in internet-based therapy procedures. [The therapeutic alliance in internet-based therapy procedures: an overview]. Nervenarzt. 2013;84:823–31. doi:10.1007/s00115-012-3659-6.
65. Ramón Fernández A de, Ruiz Fernández D, Sabuco García Y. Business Process Management for optimizing clinical processes: A systematic literature review. Health Informatics J (Health Informatics Journal). 2020;26:1305–20. doi:10.1177/
1460458219877092.
66. Butler J, Butler K, Gagne D, Hasley S, Haug P, Lario R, et al. Field Guide to Shareable Clinical Pathways: BPM+ (BPMN, CMMN & DMN) in Healthcare. 2nd ed. Milford/ MA: OMG; 2020.
67. Osterwalder A, Pigneur Y, Tucci CL. Clarifying business models: Origins, present, and future of the concept. Commun AIS (Communications of the AIS). 2005;16:1.
68. Breitschwerdt R, Heß M. Konzeption eines Bezugsrahmens zur Analyse und Entwicklung von Geschäftsmodellen mobiler Gesundheitsdienstleistungen. In: Thomas O, Nüttgens M (eds) Proceedings 4th Dienstleistungsmodellierung (DLM). Berlin: Springer. 2014; 223–243.
69. White-Williams C, Oetjen D, Williams CW. An ethical analysis of telemedicine: implications for future research. Int J Telemedicine and Clinical Practices (International Journal of Telemedicine and Clinical Practices). 2015;1:4–16. doi:10.1504/IJTMCP.2015.069470.
70. Fisk MJ. European Code of Practice for Telehealth Services. Int J Integr Care. 2013;13:T & Conf Suppl. doi:10.5334/ijic.1387.
71. Zur O. Professional Association Codes of Ethics and Guidelines On TeleMental Health, E-Therapy, Digital Ethics, & Social Media: Complete comparative list of different Codes of Ethics on a variety of topics. 2020. https://www.zurinstitute.com/ethics-of- telehealth/. Accessed 29 May 2020.
72. Liu L, Zhu D. An integrated e-service model for electronic medical records. Inf Syst E- Bus Manage. 2013;11:161–83. doi:10.1007/s10257-012-0188-6.
73. Peddle K. Telehealth in Context: Socio-technical Barriers to Telehealth use in Labrador, Canada. Computer Supported Cooperative Work. 2007; 16(6): 595–614. doi: 10.1007/s10606-006-9030-3.
74. Lin SP. Determinants of adoption of Mobile Healthcare Service. Int J Mob Commun (International Journal of Mobile Communications). 2011;9:298–315. doi:10.1504/IJMC.2011.040608.
75. Kvistgaard Jensen L, Knarvik U, Lange M, Duedal Pedersen C, Tangene W, Whitehouse D. D3.4 Personalised Blueprint for telemedicine deployment: validated and tested version: Annex 1. Brussels: Momentum/ EHTEL (European Health Telematics Association); 2015.
76. Pommer A. Avera Medical Group Pierre’s Implementation of an eConsult Program: Bringing Specialty Practices to Patients in Rural South Dakota. In: Sarnikar S, Bennett D, Gaynor M, editors. Cases on healthcare information technology for patient care management. Hershey, PA: IGI; 2013. p. 140–151. doi:10.4018/978-1-4666-2671- 3.ch009.
77. Campanella N, Morosini P, Sampaolo G, Catozzo V, Caso A, Ferretti M, et al. Medical teleconsultation to general practitioners reduces the medical error vulnerability of internal medicine patients. Eur J Intern Med (European Journal of Internal Medicine). 2015;26:675–9. doi:10.1016/j.ejim.2015.08.010.
78. German Federal Department of Health. Verordnung über das Verfahren und die Anforderungen zur Prüfung der Erstattungsfähigkeit digitaler Gesundheitsanwendungen in der gesetzlichen Krankenversicherung. Bundesanzeiger. 2020;18:768–98.
79. Baker LC, Johnson SJ, Macaulay D, Birnbaum H. Integrated Telehealth And Care Management Program For Medicare Beneficiaries With Chronic Disease Linked To Savings. Health Affair (Health Affairs). 2011;30:1689–97. doi:10.1377/hlthaff.2011.0216.
Appendix
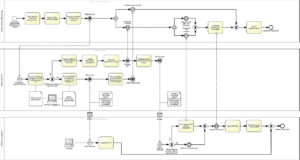
Figure A1: Usual treatment pathway for alcoholism in Germany

Figure A2: Usual treatment pathway for drug addiction in Germany
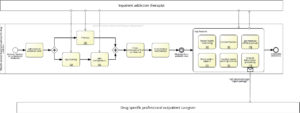
Figure A3: mHealth assisted treatment pathway for drug addiction

Figure A4: Individualized mHealth training & configuration advice
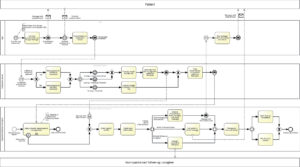
Figure A5: Health monitoring and mHealth interventions
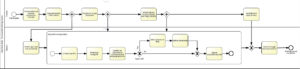
Figure A6: mHealth-based information processing
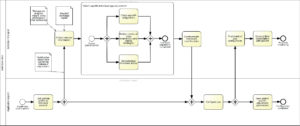
Figure A7: Synergism between IT and therapists in mHealth individualization
Footnote
iAll BPMN diagrams can be found in the Appendix.

