Pilot Study: Real-Time Monitoring and Medication Reminders in Glaucoma Patients
Alice H. Li, BA1, Yang Shou, MD1, Zhongqiu Li, MD1, Ann C. Fisher, MD1, Jeffrey L. Goldberg, MD, PhD1, YangSun, MD, PhD1, Wen-Shin Lee, MD1, Robert T. Chang, MD1,2
1Byers Eye Institute, Stanford University School of Medicine, Palo Alto, California, USA.
Corresponding Author: viroptic@gmail.com
Background: Medication Event Monitoring System (MEMS) technology in glaucoma medication adherence has not yet addressed reminder fatigue, in which patients become desensitized to reminders after repeated exposure.
Aims: To study how the prototype of a novel device, which couples real-time monitoring with custom SMS text reminders, can affect medication adherence.
Methods: We piloted a randomised prospective clinical trial, recruiting patients between June 2017 to February 2018 from the Stanford Byers Eye Institute who had been prescribed daily latanoprost for open-angle glaucoma. We monitored each participant’s usage for 13 weeks, randomising each participant into one of three arms: Arm 1 controls were monitored, Arm 2 subjects were also notified via SMS texts for missed doses, and Arm 3 subjects were monitored, notified via SMS texts for missed doses, and called monthly and/or if adherence fell below 75% to collect qualitative data on reasons for lack of adherence.
Results: Of 78 subjects who were consented, 50 subjects participated. By week 7, Arm 1 subjects had a decline in adherence compared to Arm 2, which had maintained its adherence at 78.57 ± 5.13% (p= 0.01). Arm 3 subjects maintained a steady adherence for the first 5 weeks; however, at week 6 their adherence peaked at 90.48 ± 4.12%, compared to Group 1 which had fallen to 46.43 ± 15.68% (p = 0.01).
Conclusions: Real-time custom reminders can improve glaucoma medication adherence. This pilot can aid future clinical trial design in assessing real-time electronic monitoring and custom reminders in glaucoma medication adherence.
Keywords: Adherence, Glaucoma, Reminder, Real-time, Monitoring
Introduction
Background
Medication nonadherence has become an epidemic. As of 2013, nonadherence was responsible for wasting between $100 and $300 billion in avoidable health care costs in the US annually.1 According to the WHO, approximately 50% of patients do not take their medications as prescribed.2 The detrimental ramifications of poor adherence have been studied extensively in the treatment of chronic conditions such as hypertension and glaucoma.3,4
Glaucoma is a leading cause of irreversible blindness, affecting three million Americans in 2000 with increasing prevalence every year.5 It is a chronic, largely asymptomatic, and progressive optic neuropathy leading to irreversible blindness, so early diagnosis and treatment are crucial in delaying progression of the disease6,7. There is currently no cure for glaucoma and newer methods such as neuro-regeneration are still being investigated.8,9 The most common form of glaucoma treatment is reducing intraocular pressure, which has been demonstrated to be the main modifiable risk factor in slowing disease progression.9 Glaucoma patients who do not routinely take eye drop medication may greatly increase their risk of disease progression. However, data from prior studies demonstrates that glaucoma eye drop adherence rates are 70% or less for prescribed treatment.10 As such, inadequate intraocular pressure control due to poor medication adherence is thought to be a significant cause of worsening clinical outcomes in glaucoma care and escalation of therapy.
To assess reasons for poor glaucoma medication adherence, previous studies have used Medication Event Monitoring System (MEMS) technology to compare self-reported versus monitored adherence. MEMS technology was originally developed out of growing concerns over poor medication adherence and works by electronically detecting, time-stamping, and storing data on usage of the associated medication delivery system11. In this way, MEMS differs from standard messaging-based reminder services in that it tracks medication adherence by clearly distinguishing initiation, implementation, and discontinuation of usage11. Multiple studies have found discrepancies between self-reported versus measured adherence, suggesting that electronic methods of measuring medication adherence can be more objective than inherently-biased self-reported adherence.12–16 For instance, one study found that self-reported adherence was 94% compared to 79% according to the MEMS. Further, in the same study, the independent predictors of adherence included self-efficacy, motivation, intention, cues to action, race/ethnicity, and dose frequency.17 In this sense, reasons for medication nonadherence are manifold and have been shown to include forgetfulness, low self-efficacy, difficulty with drop administration, and difficulty with medication scheduling in complicatedregimens.18–23 In light of these factors, studies on glaucoma adherence have attempted to identify innovative interventions to help patients improve adherence.
Prior studies have attempted to improve glaucoma medication adherence by designing interventions based on data collected by monitoring devices.24,25 For instance, Okeke et al. found that using weekly phone call reminders and/or audible and visible reminders on their electronic sensors resulted in improved adherence for patients with baseline poor medication adherence.13 However, this study’s device could not provide real-time reminders because its data had to be synced in clinic at the end of the study period. Consequently, the reminders were employed without real-time knowledge of medication adherence, and thus did not specifically target patients who were missing doses. This is important because reminder fatigue, in which a repeated exposure to the same alert over time desensitizes a subject to the reminder26, has been studied and reported to be both excessive and detrimental in various healthcare settings.27,28 Manifestations of harm from reminder fatigue include erroneous medication prescriptions and inappropriate dosing in computerized physician order entry systems.27,28
To our knowledge, no study using MEMS has yet designed an intervention that addresses reminder fatigue. Kali Drop (Kali Care, Santa Clara, CA) is a novel MEMS device that can monitor medication usage in real-time, as an electronic sensor sleeve that fits over an eye drop bottle.29 The version used in this study was a prototype that fit round bottles such as generic latanoprost, as opposed to cylindrical or rectangular bottles. As a prototype, the device was also novel in that it contained a cellular connection in the base unit charger and required neither Wi-Fi setup nor an app. To respect patient privacy, this prototype did not collect personal data including GPS location tracking.
We hypothesised that an intervention which reminded the patient only when a medication dose was missed could improve adherence by reducing overall reminder fatigue. The different variables tested were real-time measurements of eye drop usage without any intervention vs. real-time measurements with a smart automated reminder. In addition, to better understand the key obstacles to medication adherence when using customised phone reminders, we collected qualitative feedback by phone counseling with a third arm of study subjects who were also receiving the intervention. This phone counseling was done on a monthly basis and/or if these subjects consistently demonstrated poor adherence of less than 75%.
Methods
Trial design
We designed a pilot randomised controlled trial (RCT) consisting of three arms (see the Consolidated Standards for the Reporting of Trials [CONSORT] diagram in Figure 1). Arm 1 subjects were the control group and used the MEMS device for adherence monitoring only. Arm 2 subjects, in addition to using the device for adherence monitoring, received a standardised SMS text notification to their personal mobile phone following a missed dose. Arm 3 subjects received the same intervention as Arm 2 but also received phone counseling monthly or if adherence fell below 75% of expected doses. Arm 3 subjects also gave qualitative feedback on the device during the monthly phone calls. To perform the randomisation procedure, we used a computerised, randomly-generated list of 1, 2, or 3 to represent the three study arms. These numbers were placed in consecutively marked, sealed envelopes that were opened when we received written informed consent from a participant. Subjects were informed of all three possible study arms during the consent process and were not blinded to their assignment.

Figure 1: CONSORT Flow Diagram. Reasons subjects were lost to follow-up included: subjects were travelling and decided not to take the device with them, subjects were not accustomed to the device so stopped using it, orsubjectsstopped using the device (despite consistent signal from the device confirming the issue was not lack of signal connectivity). One subject in Arm 3 requested early discontinuation of the study due to a change in residence.
For the entrance survey, each participant reported a “medication time”, defined as the average time of day when they regularly took their medication. During the week-long baseline, subjects did not receive any notifications regardless of which study arm they had been randomised to. After the week-long baseline data collection, subjects were able to receive notifications according to their randomised study arm assignment. Subjects otherwise continued with routine follow-up and treatment for their glaucoma as a part of the standard of care and did not undergo additional testing or interventions. There was no placebo arm as all subjects received the monitoring device.
The notification schedule is outlined in Figure 2. We set the “medication window” to be at two hours before and after the medication time. For example, given a medication time of 8PM, we counted a drop as taken if it was taken two hours before the medication time or one-third of the two-hour window after the medication time (i.e. between 6PM and 8:40PM). To avoid reminder fatigue, we sent the reminder SMS text at a random time within the remaining two-thirds of the medication window after the initial third protected time. For instance, a subject with a medication time of 8PM would not receive a notification within the first third of the medication window of 2 hours (i.e. in the forty minutes between 8PM and 8:40PM), but would receive a notification at a random time in the remaining two-thirds of the medication window (i.e. in the 80 minutes between 8:40PM and 10PM). This way, subjects who regularly missed their medication time could not anticipate receiving a reminder at a set time.
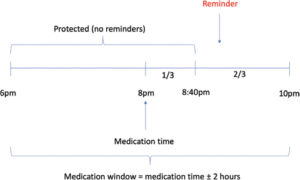
Figure 2: Reminder Configuration. In the example shown here, the subject usually expects to use their eye drops at 8PM. We used a medication window of two hours, meaning the subject was registered as adherent that day if they used their drops sometime two hours before 8PM or one-third of the two hour medication window after 8PM (i.e. between 6PM and 8:40PM). Subjects not in the control arm who missed this window received a SMS text reminder at a random time in the remaining two-thirds of the two hour medication window (i.e. in the 80 minutes between 8:40PM and 10PM).
Subjects who missed their drop and received a reminder had the chance to have their drop counted any time before midnight that day. For subjects who were travelling across time zones during the study, we asked them to notify the research team of their travel dates. This allowed the device to maintain the time of day and reminder window for which the corresponding subject was regularly expected to take their medication.
For Arm 3 subjects who received a phone call when their weekly adherence dropped below 75%, qualitative data was collected to assess reasons why subjects had decreased adherence. This included feedback on device convenience, design, and timing of reminders. The 75% threshold was chosen to recapitulate a previous study that had also defined adherence at this level.13,30
Notifications in this study were in the form of a standardised, pre-written SMS text message to the subject’s personal mobile phone. The rationale for a text message as opposed to a phone call or other means of communication was based on previous research demonstrating the ability of text messages to improve medication adherence for multiple conditions including asthma medication adherence and diabetes management.31,32 Further, studies on the effect of text messages on memory have suggested text messages can motivate behavioural change by affecting elements of cognition such as working memory.33 Subjects were also aware that any SMS text message charges would be associated with their cellular plan.
Participants
Patients were recruited from the Stanford University Department of Ophthalmology at the Byers Eye Institute. To be eligible, patients had to be receiving glaucoma care at the Byers Eye Institute, have a diagnosis of glaucoma, be at least 18 years of age, own a personal mobile phone, be able and willing to receive SMS text notifications, and have been prescribed generic latanoprost at a dosage of one drop in each eye daily for at least four months without adverse effects. The reason for generic latanoprost as opposed to other medications was to standardise for how well the device fit the bottle. We excluded patients if they were not reliable enough to operate, maintain, or keep a mobile phone, were unable to administer the eye drop due to physical limitations, had a history of eye surgery for glaucoma, had a history of very poor adherence who did not return for visits, or were concurrently enrolled in another clinical research study.
A member of the research team introduced the study to eligible consecutive patients from the clinic population during their regularly scheduled visit. Upon obtaining written informed consent, all study subjects completed an entrance survey assessing for perceived adherence, time of day they usually took their medication, and barriers to access such as cost. Collected demographic characteristics included sex, age, self-reported race, household income, and education.
Ethics Statement
The Institutional Review Board at Stanford University approved this study before its initiation. We deidentified all data according to HIPAA guidelines and adhered to all principles outlined in the Declaration of Helsinki. As all participants were already receiving the standard of care treatment, we did not need to define any stopping rules.
Device
Prior to this study, the device had already been studied for technical feasibility, had previously been studied at another institution, and at the time of this study was concurrently being used in other clinical trials commercially outside the US.29 The device is a prototype of an add-on electronic sensor that monitors eye drop bottle usage. It attaches over an existing eye drop bottle, in which the bottom of the sleeve carries the electronic hardware and the side of the sleeve facilitates the subject’s ability to squeeze the eye drop bottle. Further, the sleeve is customised to fit the shape of the particular eye drop bottle.
When trigged by bottle movement, the device sends anonymous usage data to an external cloud server. An algorithm determines usage pattern to track both the pressure on the sides of the bottle and the orientation of the bottle itself, so that the device is able to measure the number of drops squeezed out of the bottle according to how accurately both the pressure and orientation of the bottle match the pre-set algorithm. If the algorithm is not matched, the device interprets this as the subject not having used one drop in each eye that day. In this case, an automated reminder system is triggered for subjects to receive a standardised, pre-written text message notification on their mobile phone (“Reminder: take glaucoma medication”).
Outcomes
The primary end point was adherence to eye drop medication use, defined by the proportion of prescribed doses that had been taken each day over the total number of days of study participation. Each day was a binary data point; either two eye drops (presumably one per each eye) had been taken or not. Finally, this was within a window of 2 hours before or after each subject’s set medication time (see Trial Design above). For instance, within a week, adherence was calculated as the number of days medication had been registered as taken over 7 days. We analysed quantitative data collected by the device by comparing the average medication adherence (%) of the baseline first week vs the final 13th week. To gain a visual sense of adherence trends, we also analysed the average adherence per week. In total, we collected data points for the following timestamps: the subject’s medication time, the time at which the subject actually took the medication, the time at which the subject missed the medication, the time at which the subject was sent a reminder notification, and the time at which the subject took the medication after having received a notification.
Arm 3 subjects who received phone calls were interviewed to assess reasons for nonadherence and to receive feedback on the device. This was adapted from the Glaucoma Treatment and Compliance Assessment Tool GTCAT and a previous pilot study on health coaching in glaucoma medication adherence.34,35 Key elements of the interview were as follows: (1) we told subjects their adherence data to assess if informing them of their adherence was in itself an intervention that could change behaviour, (2) counseled them regarding questions on device usage and timing of their reminders, and (3) answered any other questions they had about their glaucoma medication regimen.
Statistical Analysis
We reported adherence as a continuous variable with comparison of means by Student’s t-test and compared variances by Fisher’s-test. To achieve 80% statistical power, our calculated target sample size was 16 subjects per arm assuming a mean adherence of 75% before intervention, improvement in adherence of 20% after intervention, and a Type 1 error of 5%. No correction was made for multiplicity.
Results
Recruitment and demographics
Demographic data is outlined in Table 1. A total of 50 out of 78 consented subjects (64%) participated in the study. Quantitative data was collected from the prototype for all subjects who participated in the study and qualitative reasons for drop out were collected and communicated to Kali. The first subject was enrolled in June 2017 and the last subject was enrolled in November 2017 with subsequent follow-up until February 2018. The subjects who finished the study (63.6 ± 12.33) were on average 8years younger than the 28 subjects who did not begin the study (71.07 ± 13.27; p = 0.03).
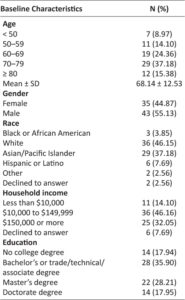
Table 1: Demographics of Baseline Characteristics(n=78)
Efficacy of the device on adherence
The primary objective of this pilot RCT was to determine if real-time monitoring coupled with custom notification reminders could improve adherence, namely by comparing the baseline versus final adherence between Arm 1 and Arm 2. We defined the baseline adherence as the average adherence of the first 7days (week 0) while the final adherence was defined as the average adherence of the last 7days (week 13). The Arm 1 baseline adherence was 75.89 ± 7.11% versus the final adherence was 54.76± 14.95%, representing a 28% decrease from baseline. By comparison, the Arm 2 baseline adherence was 78.57 ± 6.76% versus the final adherence was 60.71± 13.70%, representing a 23% decrease from baseline. Finally, the Arm 3 baseline adherence was 72.32 ± 8.60% versus the final adherence was 66.67 ± 7.06% adherence, representing only an 8% decrease from baseline. Nevertheless, none of the changes between baseline and final adherence were different between Arms 1 and 2 (p=0.12) or Arms 1 and 3 (p= 0.34).
To gain a visual understanding of adherence as subjects progressed through the study, we outlined weekly adherence trends (Figure 3). Arm 1 subjects had an initial decline in adherence, reaching its lowest adherence of 42.86 ± 14.03% at 7 weeks, down from its baseline adherence of 75.89 ± 7.11%. This marked a statistically significant difference from Arm 2, which at the 7th week had maintained its adherence at 78.57 ± 5.13% (p=0.01). Further, the difference in variance among all weekly adherence values between Arms1 and 2 was significant, with 102.21 for Arm 1 versus 32.92 for Arm 2 (F-test, p=0.05). After the 7week mark, Arm 1 adherence appeared to increase to the same adherence as Arm 2 subjects throughout the remainder of the study. However, the apparent increase in adherence for Arm 1 between weeks 7 and 12 was confirmed to be due to selection for subjects who remained in the study and were consistently adherent anyway.
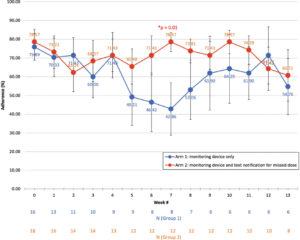
Figure 3: Weekly Adherence Trends Between Arms 1 and 2. Arm 1 subjects had an initial decline in adherence, reaching its lowest adherence of 42.86% at 7 weeks, which increased to roughly the same adherence as Arm 2 subjects during weeks 7 to 13. Meanwhile, Arm 2 subjects maintained a relatively steady adherence. The difference in variances between the arms was significant, with 102.21 for Arm 1 versus 32.92 for Arm 2 (F-test, p = 0.05). Week 7 also had a significant difference in mean adherence, with Arm 1 at 42.86 compared to Arm 2 at 78.57 (Student’s t-test, p = 0.01). To account for weekly drop off, the remaining number of subjects in each arm is shown per week along the x-axis.
Similarly, a comparison between Arms 1 and 3showed significant differences at weeks 6 and 7 (Figure 4). Specifically, Arm 3 adherence peaked at 90.48 ± 4.12% compared to Group 1 at 46.43 ± 15.68% (p = 0.01). The following week, this difference maintained statistical significance with Arm 3adherence at 82.14 ± 6.47% versus Arm 1 adherence at 42.86 ± 14.03% (p = 0.02). To determine if the additional phone calls for group 3 accounted for this difference, we compared adherence between Arm 2 and Arm 3 and found no statistical difference over all weeks of the study (lowest p = 0.15; Supplementary Figure 1).

Figure 4: Weekly adherence trends between Arms 1 and 3. There was a significant difference in Arm 1 versus Arm 3 adherence at two different weeks in the middle of the study. First, at week 6 when Arm 1 adherence was 46.43 versus Arm 3 adherence was 90.48 (Student’s t-test, p = 0.01) and second, at week 7 when Arm 1 adherence was 42.86 versus Arm 3 adherence was 82.14 (Student’s t-test, p = 0.02). There was no significant difference in the variance between Arms 1 and 3; variance of Arm 1 was 102.21 while variance of Arm 3 was 55.98 (F test, p = 0.29). Yellow arrows indicate timing of when monthly phone calls were made for Arm 3 subjects. To account for weekly drop off, the remaining number of subjects in each arm is shown per week along the x-axis.
Impact of the device on participants with full completion of the study
A comparison between Arm 1 and 2 subjects who did not drop out at any point during the study demonstrated no significant difference in adherence across all thirteen weeks of the study (lowest p=0.08; Supplementary Figure 2). Nor was there a significant difference in the variance between groups 1 and 2; the variance of group 1 was 171.22 while the variance of group 2 was 72.19 (p = 0.13).
Discussion
This study assessed if real-time custom notifications provided an intervention that could improve medication adherence. In accordance, we found that subjects receiving real-time custom notifications (Arm2) were able to maintain their baseline adherence over a longer time span in the thirteen-week study compared to the control group (Arm 1). Although comparing baseline versus final week adherence demonstrated no statistically significant difference between Arms 1 and 2, this approach may not have been sensitive enough to discern a significant difference. We reasoned this could have been due to attrition bias, as the subjects who were likely to drop out early were also likely to have worse adherence and that further, this phenomenon may not have occurred equally among the three arms. In retrospect, the effects of attrition bias could have been minimized with factors such as a larger sample size and a standardized protocol for home setup.
In line with a previous study that analysed adherence via visual representation of adherence data and identified four easily-defined patterns of adherence (good adherence, discontinued usage, frequent drug holidays, and frequent missed doses with low adherence rates), we graphed weekly adherence to assess how usage trends differed among our three study arms.14 Indeed, by week 7 the intervention had led to a significant difference in adherence between Arms 1 and 2, with Arm 1’s graphical representation most closely resembling increasingly frequent missed doses with each week while Arm 2’s graphical representation most closely represented good adherence. This was supported by a similar finding when the control arm (Arm 1) was compared to the group that, in addition to receiving texts for missed doses, also received monthly phone calls and/or if adherence fell below 75% (Arm 3). This difference was likely driven primarily by the custom text notifications instead of phone calls, as there was no difference in adherence across all weeks of the study between Arms 2 and 3 (lowest p = 0.15).
Further, when we analysed the average adherence of the subjects who completed the full thirteen-week study, we found no significant difference in adherence between Arms 1 and 2 (p = 0.08, Supplementary Figure 2). This suggests that the subjects who had the perseverance to use the device throughout the study period were going to maintain their adherence regardless of whether they received reminders or not. This suggests this subgroup of study-compliant individuals were able to maintain their adherence through behavioural mechanisms that do not necessarily require reminders but rather interventions such as education and environmental restructuring.36 For instance, several compliant subjects qualitatively reported not believing that they relied on the device’s notifications because their eye drops were left next to their toothbrush or glasses, i.e. associated with an object they used every day. Furthermore, home setups of the devices were not standardized which may have contributed to a reporting error or measurement error in the data.
Demographically, the subjects who finished the study were on average 63.6 years old, which was approximately 8 years younger than the subjects who consented to but did not begin the study (p=0.03; Table 1). Possible reasons include that older patients were less willing to participate in a study and/or were less able to use the device due to ongoing conditions such as arthritis or dementia. Although this is consistent with prior work that did not find adequate evidence showing that elderly patients have poorer adherence than younger patients,37 in a previous study conducted by Kali Care with their monitoring device, adherence did tend to increase with age (r = 0.673, p <0.001).29 Further investigation of glaucoma medication adherence in elderly patients may require clarifying differences between elderly patients who manage their own care versus those who rely on younger caretakers or family members.
Limitations of this study included the small sample size which may have precluded the achievement of statistical significance in baseline versus final week adherence among the study arms. Another limitation was the prototype was designed to only fit bottles of generic latanoprost, which limited the number of eligible subjects for the study and could have led our study to select for subjects of a given socioeconomic status. Further, the prototype’s inability to automatically update the system to account for a change in time zone if a subject traveled; for this study we could only update time zones manually for patients who told us of their travel plans. A possible solution could be installing GPS tracking in future versions to automatically maintain customised reminders across time zones, although this innovation would need to be balanced with each subject’s permission for tracking their location. In addition, while the Hawthorne effect on medication usage is a consideration in any electronic monitoring of medication usage, it was of less concern for this study because both our control and intervention subjects were aware of being monitored.38 A previous study of medication usage in glaucoma patients supports this point, as they compared open versus masked monitoring and found no significant difference.39
Future work includes running a clinical trial with a larger sample size to further validate the findings of this pilot study. Additional features of this study should include broadening the medication window from two hours to a more forgiving time window and introducing personalised health coaching at the start of the study, which has been shown to improve medication adherence not only for glaucoma13 but also for other chronic conditions including Type 2 diabetes mellitus40 and chronic obstructive pulmonary disease.41 Finally, a more focused way to prevent reminder fatigue could involve incorporating artificial intelligence (AI) technology for writing unique SMS texts that never repeat the same message and are personalized to each subject.
Conclusions
This randomised clinical trial piloted an innovative prototype that provided real-time monitoring coupled with custom notifications and found that subjects receiving these notifications were able to maintain their baseline adherence over a longer time span than the control group. Future studies may aim to include a randomized clinical trial with a larger sample size and varied reminders with more customized or dynamic parameters.
Acknowledgements
The authors would like to thank Kali Care for providing the devices used in this study. The authors and this study are supported by the National Eye Institute (NEI) for their P30 Center Core Grant for the Stanford Vision Research Core (EY026877) and to the Research to Prevent Blindness (RPB) institutional grant for the Stanford University School of Medicine.
Declaration of Competing Interests
All authors have completed the Unified Competing Interest form. RTC is an advisor to Kali Care which supplied the devices for this research; all authors declare no other conflicts of interest including no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
1. Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35–44. doi: 10.2147/RMHP.S19801
2. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. doi: 10.4065/mcp.2010.0575
3. Ross S, Walker A, MacLeod MJ. Patient compliance in hypertension: role of illness perceptions and treatment beliefs. J Hum Hypertens. 2004;18(9):607–613. doi: 10.1038/sj.jhh.1001721
4. Reardon G, Kotak S, Schwartz G. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer Adherence. 2011;5:441. doi: 10.2147/PPA.S23780
5. Friedman DS, Wolfs RCW, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol (Chicago, Ill 1960). 2004;122(4):532–538. doi: 10.1001/archopht.122.4.532
6. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192
7. Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol (Chicago, Ill1960). 2003;121(1):48–56. http://www.ncbi.nlm.nih.gov/pubmed/12523884. Accessed February 10, 2019.
8. Chang EE, Goldberg JL. Glaucoma 2.0: Neuroprotection, Neuroregeneration, Neuroenhancement. Ophthalmology. 2012;119(5):979–986. doi: 10.1016/j.ophtha.2011.11.003
9. Lusthaus J, Goldberg I. Current management of glaucoma. Med J Aust. February 2019. doi: 10.5694/mja2.50020
10. Cramer JA, Spilker B. Patient Compliance in Medical Practice and Clinical Trials. Raven Press; 1991. https://books.google.com/books?id=vDBrAAAAMAAJ&q=Validity+of+standard+compliance+measures+in+glaucoma+compared+with+an+electronic+eye+drop+monitor.&dq=Validity+of+standard+compliance+measures+in+glaucoma+compared+with+an+electronic+eye+drop+monitor.&hl=en. Accessed February 10, 2019.
11. El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol. 2016;82(1):268–279. doi: 10.1111/bcp.12942
12. Cate H, Bhattacharya D, Clark A, Fordham R, Holland R, Broadway DC. Improving adherence to glaucoma medication: a randomised controlled trial of a patient-centred intervention (The Norwich Adherence Glaucoma Study). BMC Ophthalmol. 2014;14(1):32. doi: 10.1186/1471-2415-14-32
13. Okeke CO, Quigley HA, Jampel HD, et al. Interventions Improve Poor Adherence with Once Daily Glaucoma Medications in Electronically Monitored Patients. Ophthalmology. 2009;116(12):2286–2293. doi: 10.1016/j.ophtha.2009.05.026
14. Ajit RR, Fenerty CH, Henson DB. Patterns and rate of adherence to glaucoma therapy using an electronic dosing aid. Eye (Lond). 2010;24(8):1338–1343. doi: 10.1038/eye.2010.27
15. Hein AM, Rosdahl JA, Bosworth HB, et al. The Relationship of Self-Report and Medication Possession With Glaucoma Medication Administration Success. J Glaucoma. 2019;28(3):e46-e48. doi: 10.1097/IJG.0000000000001136
16. Kass MA, Meltzer DW, Gordon M. A Miniature Compliance Monitor for Eyedrop Medication. Arch Ophthalmol. 1984;102(10):1550–1554. doi: 10.1001/archopht.1984.01040031266033
17. Cook PF, Schmiege SJ, Mansberger SL, Kammer J, Fitzgerald T, Kahook MY. Predictors of Adherence to Glaucoma Treatment in a Multisite Study. Ann Behav Med. 2015;49(1):29–39. doi: 10.1007/s12160-014-9641-8
18. Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to Medications: Insights Arising from Studies on the Unreliable Link Between Prescribed and Actual Drug Dosing Histories. Annu Rev Pharmacol Toxicol. 2012;52(1):275–301. doi: 10.1146/annurev-pharmtox-011711-113247
19. Vrijens B, Urquhart J. Methods for measuring, enhancing, and accounting for medication adherence in clinical trials. Clin Pharmacol Ther. 2014;95(6):617–626. doi: 10.1038/clpt.2014.59
20. Newman-Casey PA, Robin AL, Blachley T, et al. The Most Common Barriers to Glaucoma Medication Adherence: A Cross-Sectional Survey. Ophthalmology. 2015;122(7):1308–1316. doi: 10.1016/j.ophtha.2015.03.026
21. Hennessy AL, Katz J, Covert D, Protzko C, Robin AL. Videotaped Evaluation of Eyedrop Instillation in Glaucoma Patients with Visual Impairment or Moderate to Severe Visual Field Loss. Ophthalmology. 2010;117(12):2345–2352. doi: 10.1016/j.ophtha.2010.03.040
22. Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in Glaucoma: Objective Measurements of Once-Daily and Adjunctive Medication Use. Am J Ophthalmol. 2007;144(4):533–540.e2. doi: 10.1016/j.ajo.2007.06.012
23. Stone JL, Robin AL, Novack GD, Covert DW, Cagle GD. An Objective Evaluation of Eyedrop Instillation in Patients With Glaucoma. Arch Ophthalmol. 2009;127(6):732. doi: 10.1001/archophthalmol.2009.96
24. Boland M V., Chang DS, Frazier T, Plyler R, Jefferys JL, Friedman DS. Automated Telecommunication-Based Reminders and Adherence With Once-Daily Glaucoma Medication Dosing. JAMA Ophthalmol. 2014;132(7):845. doi: 10.1001/jamaophthalmol.2014.857
25. Beckers HJM, Webers CAB, Busch MJWM, Brink HMA, Colen TP, Schouten JSAG. Adherence improvement in Dutch glaucoma patients: a randomized controlled trial. Acta Ophthalmol. 2013;91(7):610–618. doi: 10.1111/j.1755-3768.2012.02571.x
26. Ancker JS, Edwards A, Nosal S, et al. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):36. doi: 10.1186/s12911-017-0430-8
27. Seidling HM, Klein U, Schaier M, et al. What, if all alerts were specific – Estimating the potential impact on drug interaction alert burden. Int J Med Inform. 2014;83(4):285–291. doi: 10.1016/j.ijmedinf.2013.12.006
28. Stultz JS, Nahata MC. Appropriateness of commercially available and partially customized medication dosing alerts among pediatric patients. J Am Med Informatics Assoc. 2014;21(e1):e35-e42. doi: 10.1136/amiajnl-2013-001725
29. Gatwood JD, Johnson J, Jerkins B. Comparisons of Self-reported Glaucoma Medication Adherence With a New Wireless Device: A Pilot Study. J Glaucoma. 2017;26(11):1056–1061. doi: 10.1097/IJG.0000000000000777
30. Sleath B, Blalock SJ, Carpenter DM, et al. Ophthalmologist-patient communication, self-efficacy, and glaucoma medication adherence. Ophthalmology. 2015;122(4):748–754. doi: 10.1016/j.ophtha.2014.11.001
31. Riley WT, Rivera DE, Atienza AA, Nilsen W, Allison SM, Mermelstein R. Health behavior models in the age of mobile interventions: are our theories up to thetask? Transl Behav Med. 2011;1(1):53–71. doi: 10.1007/s13142-011-0021-7
32. Johnson KB, Culpepper D, Scott P, Gordon JS, Harris C. The utility of providing automated medication dose reminders to young children on chronic medication. J Telemed Telecare. 2011;17(7):387–391. doi: 10.1258/jtt.2011.110314
33. Buehlmann A, Deco G. The Neuronal Basis of Attention: Rate versus Synchronization Modulation. J Neurosci. 2008;28(30):7679–7686. doi: 10.1523/JNEUROSCI.5640-07.2008
34. Rosdahl J, Vin A, Schneider S, Muir K. Health coaching for glaucoma care: a pilot study using mixed methods. Clin Ophthalmol. 2015;9:1931. doi: 10.2147/OPTH.S92935
35. Barker GT, Cook PF, Schmiege SJ, Kahook MY, Kammer JA, Mansberger SL. Psychometric Properties of the Glaucoma Treatment Compliance Assessment Tool in a Multicenter Trial. Am J Ophthalmol. 2015;159(6):1092–1099.e2. doi: 10.1016/j.ajo.2015.03.006
36. Crayton E, Wright AJ, Ashworth M. Improving medication adherence in stroke survivors: the intervention development process. BMC Health Serv Res. 2018;18(1):772. doi: 10.1186/s12913-018-3572-1
37. Broadway DC, Cate H. Pharmacotherapy and Adherence Issues in Treating Elderly Patients with Glaucoma. Drugs Aging. 2015;32(7):569–581. doi: 10.1007/s40266-015-0282-9
38. Pan S-C, Tien K-L, Hung I-C, et al. Compliance of Health Care Workers with Hand Hygiene Practices: Independent Advantages of Overt and Covert Observers. Kluytmans J, ed. PLoS One. 2013;8(1):e53746. doi: 10.1371/journal.pone.0053746
39. Hermann MM, Papaconstantinou D, Muether PS, Georgopoulos G, Diestelhorst M. Adherence with brimonidine in patients with glaucoma aware and not aware of electronic monitoring. Acta Ophthalmol. 2011;89(4):e300-e305. doi: 10.1111/j.1755-3768.2010.02050.x
40. Wayne N, Perez DF, Kaplan DM, Ritvo P. Health Coaching Reduces HbA1c in Type 2 Diabetic Patients From a Lower-Socioeconomic Status Community: A Randomized Controlled Trial. J Med Internet Res. 2015;17(10):e224. doi: 10.2196/jmir.4871
41. Tülüce D, Kutlutürkan S. The effect of health coaching on treatment adherence, self-efficacy, and quality of life in patients with chronic obstructive pulmonary disease. Int J Nurs Pract. 2018;24(4):e12661. doi: 10.1111/ijn.12661

